
Desk Report
Publish: 12 Jan 2022, 04:12 pm
Covid-19 Treatment
Radiant Pharmaceuticals Ltd. organizes a scientific seminar on monoclonal antibodies. Photo: Shampratik Desakal
The global pandemic infection is once again on the rise. At the same time, the number of patients suffering from covid-19 in Bangladesh is increasing at an alarming rate, reports a press release.
In this context, the effective treatment of high-risk covid patients is monoclonal, a drug approved worldwide by the United Kingdom's Medicines and Healthcare Products Regulatory Authority (MHRA) and Japan, and by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Casirivimab & Imdevimab monoclonal antibody injection by F. Hoffmann-La Roche Ltd, Switzerland has recently been approved by the Department of Drug Administration in Bangladesh for emergency marketing of high-risk corona patients.
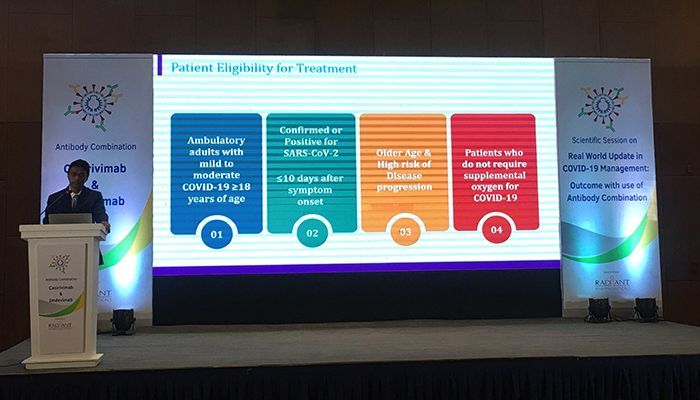
On Wednesday (January 12), Radiant Pharmaceuticals Limited organized a scientific seminar on monoclonal antibodies. In this seminar organized at Hotel Intercontinental in the capital, the eminent doctors of the country gave important discussions about the present and future medical methods.
With the use of this drug and the provision of proper medical care, the condition of a number of high-risk corona patients has been alleviated before it became critical and the risk of death has been reduced. As a result, hospital admissions of covid patients using those antibodies have dropped by about 70 percent.
Clinical benefits
Recently, the Department of Drug Administration in Bangladesh has approved Casirivimab and Imdevimab for use in the treatment of high-risk corona patients and for emergency marketing. Following this, Radiant Pharmaceuticals Limited has been successfully importing these medicines into a cold chain system to ensure the quality of these medicines in Bangladesh.
This initiative has brought a new weapon among physicians to combat the Corona pandemic, the use of which has significantly improved the quality of medical services. As a result of timely steps taken by the Government of Bangladesh, the Covid-19 outbreak is largely controlled in Bangladesh today.
Radiant Pharmaceuticals Limited is proud to be a partner in this initiative. This achievement is due to the tireless work of all the employees of Radiant. Radiant is committed to continuing working with all such veterans in the face of such great initiatives and epidemics of the Government of Bangladesh in the future as well.
Emergency Marketing Approval /
Registration:
Subscribe Shampratik Deshkal Youtube Channel
© 2024 Shampratik Deshkal All Rights Reserved. Design & Developed By Root Soft Bangladesh