
Desk Report
Publish: 20 Jun 2020, 04:33 am
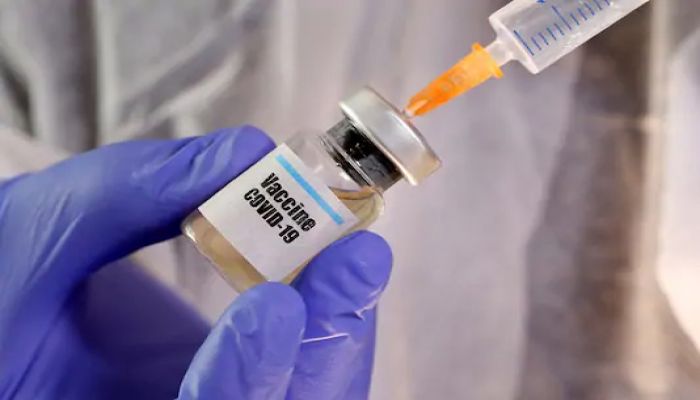
China's Sinovac Biotech Ltd stated that it expected the final phase of its prospective COVID-19 vaccine to be completed by November this year, claiming that the inoculation was shown to be "safe and effective" for humans so far, US-based ABC News reported yesterday.
“Currently, we are conducting the trial on humans and we expect some preliminary data for Phase I and Phase II to be available within this month,” the report said quoting Sinovac’s senior investor relations director Helen Yang as saying. She told the ABC that the company was following the same three-phase testing protocol used in western countries and so far they tested 144 volunteers in phases I and 600 in phase II.
“The vaccine shows a good safety profile . . . we haven’t seen any severe adverse reaction after using our vaccines,” Yang claimed adding the vaccine could finish phase III trial by the fall.
She said phase III trial needs to exhaust a few months for review of phase II results when “we will know how much of the antibody level can be induced by the vaccine”.
“And then phase III will tell you if the level of antibodies will provide protection,” Yang explained.
Sinovac, however, is yet to update its website the latest development while the ABC said it was the first US news outlet to have access to the Chinese company’s COVID-19 vaccine study.
The study on the production of inoculation in China came a day after the World Health Organization (WHO) announced that millions of vaccine doses were needed to be produced by the current year and 2 billion by 2021.
The WHO also hoped that the initial doses would be targeted to the most vulnerable people while the world’s leading drug companies, universities, and governments are racing to develop a vaccine or vaccines against the coronavirus and halt the global pandemic. WHO’s chief scientist Soumya Swaminathan said researchers were working on more than 200 vaccine candidates around the world while 10 of those were in human testing and “if we’re very lucky, there will be one or two successful candidates before the end of this year”.
She identified three groups most in need of the first wave of vaccine doses saying “you have to start with the most vulnerable and then progressively vaccinate more people”.
Swaminathan categorized groups as — high-exposure front-line workers, such as physicians and police officers; those most vulnerable to disease, such as the elderly and diabetics; and people in high-transmission settings, such as urban slums and care homes.
According to WHO, Sinovac, which has a history of vaccine development, is one of at least 11 approved bodies worldwide to carry out a human inoculation trial.
Earlier this week, Sinovac revealed a decision to perform a phase III clinical trial in Brazil, where infections are on the rise — an indication that the vaccine may be on target for early phase III trials.
However, US top infectious disease expert Dr. Anthony Fauci tended to be wary of the prospective Chinese vaccine by saying that regulators in the United States and Europe have the most stringent safety standards in the world for testing and developing new drugs and vaccines.
“I certainly don’t have as much confidence in what comes out of China than I do which comes out of the United States,” Fauci said in an interview with ABC News.
The US drug maker Moderna and the UK's Oxford University, funded by British drug maker AstraZeneca, are two significant precursors to the production of COVID-19 vaccines, presently undertaking phase II human inoculation trials.
But in another media interview Fauci, however, said just because the Moderna vaccine and another produced by Oxford University appeared to be “temporally ahead,” it did not mean their final results would be the best.
Wealthy countries are placing orders for treatment before it is even approved as the vaccine race intensifies, leaving questions as to whether developing nations will get any vaccines in time to save lives.
The British government said that if the Oxford vaccine proved effective, the first 30 million doses would be for Britons while AstraZeneca separately signed deals to make at least 300 million doses available for the US and another 400 million for the European Union members.
Beijing earlier promised to make universal its prospective vaccines while Yang said the company was unlikely to keep the vaccines for China alone.
“We are talking to different — many countries, they’re also discussing about doing the trials and then how to secure the using of vaccines to help them,” Yang said but could not assure when the vaccine would be available to the general public.
Sinovac claimed it was able to manufacture 100 million doses, although it appeared unclear how easily many countries would have exposure to the vaccine if it was effective, as China's own population reportedly stands at almost 1.4 billion.
UN trade and development agency UNCTAD recently said poorer countries would need to boost local productions to ensure availability of prospective COVID-19 vaccines amid concerns over “who would and would not have access to the shot, if and when one (of the vaccines) is approved”.
“Once a vaccine for COVID-19 is available, the massive demand is likely to outstrip supply quickly,” UNCTAD’s director of investment and enterprise James Zhan said while opening an online seminar with World Health Organization (WHO) three weeks ago.
The UNCTAD statement indicated that the UN Organization has partnered up with the WHO to search at ways to adapt to the need for developing countries to access critical COVID-19 drugs, including prospective vaccines.
Source: BSS
Subscribe Shampratik Deshkal Youtube Channel
© 2024 Shampratik Deshkal All Rights Reserved. Design & Developed By Root Soft Bangladesh